Evaluating the Efficacy of Rapid Antigen Nasal Swab Test in Low Prevalence SARS-CoV-2 Settings
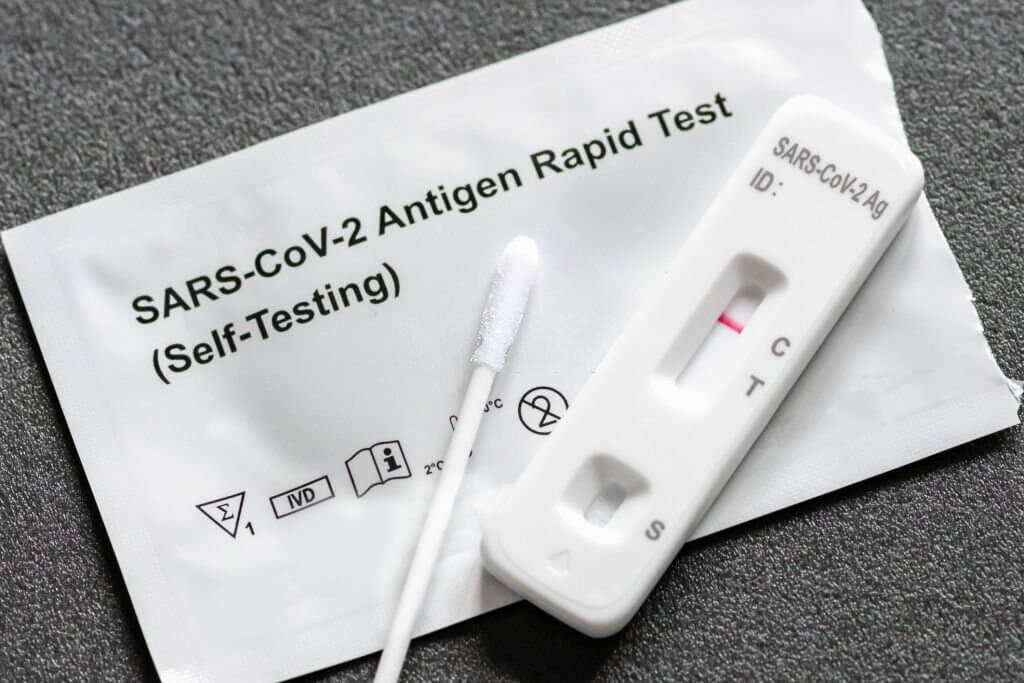
In a significant new study examining the efficacy of rapid antigen (Ag) tests in detecting SARS-CoV-2, researchers have evaluated the accuracy of using nasal swabs in a low prevalence setting. This approach, which has not been studied extensively before, may offer new insights into more effective, large-scale screening protocols for COVID-19.
The researchers conducted the study in a public setting where the prevalence of SARS-CoV-2 was as low as 0.9%. The rapid Ag test, combined with a RT-PCR test, was used as a broad screening tool within the general population, which makes the findings of the study highly generalizable. Notably, a negative Ag test performed within the last 72 hours could serve as a qualifier for a COVID passport, or Digital Green Certificate.
The results of the study revealed a sensitivity of 48.5% and a specificity of 100% for the Ag test when performed on anterior nasal swabs. Given the low prevalence in the study population, the sensitivity of the Ag test must be interpreted carefully. Despite the relatively lower sensitivity, the Ag test's value in screening remains significant, particularly if the testing frequency is high.
The researchers suggest that even though the Ag test has lower sensitivity, it remains a valuable tool for mass screening, considering the ease of sample collection, quick results, and lower costs. The test's efficiency lies particularly in its ability to detect high viral loads, which are associated with greater transmissibility.
While RT-PCR remains the gold standard for SARS-CoV-2 detection, no diagnostic test is without faults. In this study, 1.4% of the RT-PCR tests resulted in missing test results due to registration errors and the necessity of transporting samples to laboratories for testing. In contrast, all participants received Ag test results before leaving the test facility.
Importantly, the choice of the RT-PCR test as a reference and the defined criteria for positive results can significantly affect the sensitivity and specificity of the investigated test. In this study, adjusting the criteria for a positive RT-PCR result from a Ct value of ≤ 38 to ≤ 33 and ≤ 30 increased the sensitivity of the Ag test to 56.2% and 63.9%, respectively.
The study also proposes that anterior nasal swabs could pave the way for self-collected sampling and home testing. However, the technique used for collection can impact the test's sensitivity, underscoring the need for clear collection instructions.
Finally, the study compares the results obtained using the Ag test in a previous high-prevalence setting to the current low-prevalence setting. Interestingly, despite a 20 percentage point difference in the sensitivity of the test method due to differing prevalence rates, the sensitivity of the Ag test remained the same for asymptomatic individuals, regardless of whether the samples were collected from the nasopharynx or the anterior part of the nose.
Despite certain limitations, this study provides valuable insight into the potential role of the Ag test in low-prevalence, mass screening settings, suggesting that despite lower sensitivity, the test can be a valuable tool in SARS-CoV-2 screening if testing is frequent. This reinforces the idea that the Ag test can be a beneficial supplement to RT-PCR testing in a comprehensive public SARS-CoV-2 screening strategy.
Reference
Jakobsen KK, Jensen JS, Todsen T, Kirkby N, Lippert F, Vangsted AM, Klokker M, von Buchwald C. Accuracy of anterior nasal swab rapid antigen tests compared with RT-PCR for massive SARS-CoV-2 screening in low prevalence population. APMIS. 2022 Feb;130(2):95-100. doi: 10.1111/apm.13189. Epub 2021 Dec 6. PMID: 34758150; PMCID: PMC8652940.
Related Posts
How Accurate Are Nasal Swab Rapid Tests For COVID-19?
7 Essential Applications of Nasal Swabs You Should Know