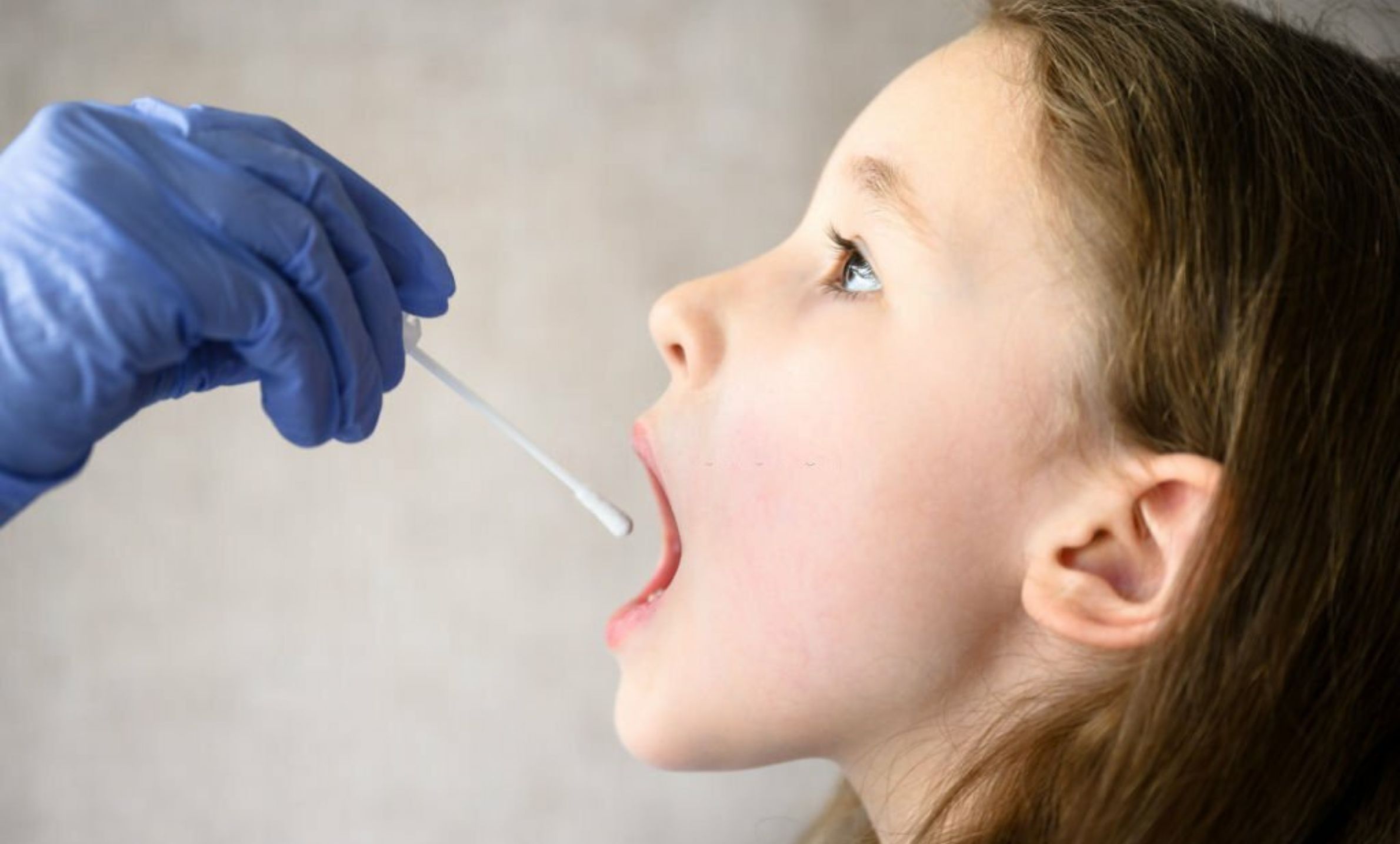
SPECIMEN COLLECTION SWABS
Specimen Collection Swabs support in vitro diagnostic sampling for oral and nasal use, with customization options.

VIRUS TRANSPORT MEDIUM
Virus Transport Medium Kits enable rapid epidemic virus sampling, available in customizable quantities.

SALIVA COLLECTION KITS
Saliva Collection Kits enable painless, easy sample collection for at-home use.
.jpg?x-oss-process=image/resize)
CHG SWABS
2% CHG and 70% IPA individually packaged disinfectant eliminates bacteria and reduces puncture infection risk.

02-26-2025
Understanding the Difference Between Influenza A and B
Understanding the Difference Between Influenza A and B I. Overview of Seasonal Influenza Influenza, commonly known as the flu, affects millions of people each year during fall and winter seasons. According to CDC data between 2010-2023, flu has caused up to 41 million illnesses, 710,000 hospitalizations, and 51,000 deaths annually in the United States. While many people recover quickly, older adults and those with chronic conditions face higher risks of severe complications. Flu Season Statistics (2010-2023) Annual Impact Illnesses Up to 41 million Hospitalizations Up to 710,000 Deaths Up to 51,000 Peak Season October through May II. Types of Influenza Viruses There are four types of influenza viruses: A, B, C, and D. Types A and B are responsible for seasonal flu epidemics in humans. Type C only causes mild illness and isn't associated with outbreaks, while type D primarily affects cattle and doesn't appear to infect humans. Influenza Type Affects Humans Causes Epidemics Severity Type A Yes Yes Moderate to severe Type B Yes Yes Mild to moderate Type C Yes No Mild only Type D No No N/A (cattle only) III. Key Differences Between Flu A and B Influenza A accounts for approximately 75% of all flu cases and can infect humans, birds, and mammals. It mutates frequently and is typically responsible for pandemics. Influenza B only affects humans, makes up about 25% of cases, and generally causes milder illness, though it can be severe in children. Characteristic Influenza A Influenza B Prevalence ~75% of cases ~25% of cases Host range Humans, birds, mammals Humans only Subtypes Many (H1N1, H3N2, etc.) Two lineages (Victoria, Yamagata) Pandemic potential High Low Seasonal timing Early-mid season Later season (spring) Age group most affected Adults Children IV. Common Symptoms and Severity Both influenza A and B cause similar symptoms, including fever, headache, muscle aches, fatigue, cough, and sometimes respiratory distress. Type A generally causes more severe symptoms in adults, while type B can be particularly severe in children under 5 years old. Symptom Influenza A Influenza B Fever 100-102°F 100-102°F Onset Sudden Sudden Headache Common Common Body aches Severe Moderate Complications in children More ear infections More seizures, vomiting, diarrhea Overall severity in adults Higher Lower Overall severity in children Moderate Can be severe V. Treatment Options Treatment is the same regardless of which type of flu you have. Antiviral medications can reduce symptom severity and duration when taken early. Supportive care includes rest, hydration, and over-the-counter medications for fever and pain relief. Treatment For Flu A For Flu B When to Start Form Oseltamivir (Tamiflu) Yes Yes Within 48 hours Pill/liquid Zanamivir (Relenza) Yes Yes Within 48 hours Inhaled powder Peramivir (Rapivab) Yes Yes For severe cases IV injection Baloxavir (Xofluza) Yes Yes Within 48 hours Single-dose pill OTC symptom relief Yes Yes As needed Various VI. Prevention Strategies The best protection against both influenza A and B is annual vaccination. For older adults, higher-dose or adjuvanted vaccines are recommended for stronger immune response. Other preventive measures include hand hygiene, avoiding sick contacts, and mask-wearing in crowded settings during flu season. Prevention Method Effectiveness Recommended For Notes Annual flu vaccine High Everyone 6+ months Best in September/October Higher-dose vaccines Enhanced Adults 65+ Stronger immune response Hand washing Moderate Everyone 20+ seconds with soap Avoiding sick contacts High Everyone Especially important for high-risk groups Mask wearing Moderate During outbreaks In crowded/indoor settings Surface disinfection Moderate High-touch areas Virus survives 48 hours on surfaces Related posts Everything You Need To Know About Flocked Swabs
READ MORE
02-26-2025
Evaluation of Flocked Swabs for Respiratory Epithelial Cell Collection
Evaluation of Flocked Swabs for Respiratory Epithelial Cell Collection Abstract Previous research has demonstrated the superior performance of flocked swabs compared to traditional rayon swabs for collecting respiratory specimens. This study independently evaluated Mantacc flocked swabs against standard rayon swabs for respiratory epithelial cell collection efficiency. Using a methodology similar to previously published work, we examined both nasopharyngeal (NPS) and nasal swabs (NS) from 18 volunteers and 64 symptomatic patients. Mantacc flocked NPS collected significantly more respiratory epithelial cells (60.2 vs. 24.5 cells/hpf; p<0.01) than rayon swabs. Similarly, in symptomatic patients, flocked swabs yielded 65.8 cells/hpf compared to 27.6 cells/hpf with rayon swabs (p<0.001). The enhanced cell recovery was consistent across age groups and viral etiologies. Infected cell recovery was also significantly higher with flocked swabs (12.4 vs. 5.8 infected cells/hpf; p<0.001). Our findings confirm previous research demonstrating that the flocked swab design substantially improves respiratory specimen collection, which may significantly impact diagnostic sensitivity for respiratory pathogens. Introduction Accurate diagnosis of respiratory infections remains critically important for appropriate patient management, infection control, and epidemiological surveillance. The quality of the clinical specimen is a fundamental determinant of diagnostic test performance, regardless of the detection methodology employed. Previous research by Daley et al. (2006) demonstrated that flocked swab design significantly improved respiratory epithelial cell collection compared to conventional rayon swabs.Building on this important work, we sought to independently validate these findings using Mantacc flocked swabs, which employ a similar design principle with perpendicular nylon fibers creating a brush-like surface. We hypothesized that the Mantacc flocked design would demonstrate similar advantages in cell collection efficiency, potentially offering clinicians an alternative option for improved respiratory sampling. Respiratory specimens with higher epithelial cell counts provide better opportunities for detecting viral pathogens through direct fluorescent antibody (DFA) testing, nucleic acid amplification, or culture methods. Therefore, optimizing specimen collection is a logical approach to improving diagnostic sensitivity without modifying the laboratory testing procedures themselves. Materials and Methods Study Participants We recruited 18 healthy adult volunteers from laboratory and hospital staff for the comparison of swab types. For the patient component, we collected and analyzed 64 nasopharyngeal specimens from individuals presenting with respiratory symptoms (43 children, 21 adults). Patient specimens were categorized based on final diagnosis: influenza virus positive (n=22), respiratory syncytial virus (RSV) positive (n=23), and negative for respiratory viruses by DFA (n=19). Swab Comparison We compared Mantacc flocked swabs (Miraclean Technology Co., Ltd) with standard rayon swabs. The Mantacc flocked swab features nylon fibers attached perpendicularly to the plastic shaft, creating a brush-like surface designed to maximize cell collection and elution. For volunteers, we performed four separate swabbings per participant: flocked NPS, rayon NPS, flocked NS, and rayon NS, with randomized order and alternating nares. Nasopharyngeal swabs were inserted to a depth equal to the distance from the nostril to the ear lobe, while nasal swabs were inserted approximately 4-5 cm. Participants rated discomfort on a 100-mm visual analog scale.For symptomatic patients, sampling was performed as part of routine clinical care using either flocked or rayon NPS based on availability on the ward. All samples were collected by trained nursing staff and transported in universal transport medium. Laboratory Processing All specimens were processed using standardized protocols for DFA testing. After vortexing for 20 seconds to release collected cells, the transport medium was centrifuged, and cell pellets were resuspended. Slides were prepared, fixed, and stained with fluorescein-labeled monoclonal antibodies against common respiratory viruses. Cell counts were performed by two independent microscopists blinded to swab type, with respiratory epithelial cells (both infected and uninfected) counted per high-power field (hpf) at 400× magnification. Ten fields were examined per slide, and the average count was calculated. Statistical Analysis Cell count data were log-transformed to improve normality. Comparisons were made using paired t-tests for volunteer samples and unpaired t-tests for patient samples. Multivariable linear regression models were used to adjust for potential confounding factors including age group, viral etiology, and symptom duration. Statistical significance was set at p<0.05. Results Volunteer Sampling Among volunteers, Mantacc flocked NPS collected significantly more respiratory epithelial cells than rayon NPS (geometric mean 60.2 vs. 24.5 cells/hpf; p<0.01). Similarly, flocked NS yielded more cells than rayon NS (32.8 vs. 16.3 cells/hpf; p<0.01). Interestingly, flocked NS performance approached that of rayon NPS, suggesting potential utility of the less invasive approach when using flocked swabs. Discomfort scores were slightly higher for flocked NPS (mean VAS 59.7 mm) compared to rayon NPS (mean VAS 45.1 mm; p=0.08), though this difference did not reach statistical significance. No significant difference in discomfort was reported between flocked and rayon NS (p=0.52). Patient Sampling In symptomatic patients, Mantacc flocked swabs collected a mean of 65.8 respiratory epithelial cells/hpf compared to 27.6 cells/hpf for rayon swabs (mean difference 38.2 cells; 95% CI: 28.7-47.7; p<0.001). This advantage was maintained across all subgroups: Children: Flocked swabs yielded 68.1 cells/hpf vs. 22.4 cells/hpf for rayon (p<0.001) Adults: Flocked swabs yielded 60.4 cells/hpf vs. 28.9 cells/hpf for rayon (p<0.001) Influenza-positive: Flocked swabs yielded 69.8 cells/hpf vs. 31.2 cells/hpf for rayon (p<0.001) RSV-positive: Flocked swabs yielded 53.2 cells/hpf vs. 20.1 cells/hpf for rayon (p<0.001) Virus-negative: Flocked swabs yielded 79.5 cells/hpf vs. 25.7 cells/hpf for rayon (p<0.001) Importantly, the number of infected cells detected was also significantly higher with flocked swabs. Among influenza-positive patients, flocked swabs detected a mean of 16.7 infected cells/hpf compared to 7.5 cells/hpf with rayon swabs (p<0.001). For RSV-positive patients, flocked swabs detected 31.4 infected cells/hpf versus 11.7 cells/hpf with rayon swabs (p<0.001). After adjusting for age, viral etiology, and symptom duration in multivariable regression analysis, the advantage of flocked swabs remained substantial and statistically significant (adjusted mean difference of 39.8 cells/hpf; 95% CI: 29.4-50.2; p<0.001). Discussion Our findings strongly validate previous research by Daley and colleagues demonstrating the superior collection efficiency of flocked swabs compared to traditional rayon swabs for respiratory specimens. The Mantacc flocked swabs evaluated in our study showed a remarkably similar 2-3 fold improvement in epithelial cell yield, suggesting this is a genuine advantage of the flocked design rather than a brand-specific phenomenon. The clinical implications of these findings are substantial. Improved specimen quality directly impacts diagnostic sensitivity, particularly for tests like DFA that rely on detection of infected cells. The increased yield of both total and infected epithelial cells with flocked swabs provides testing laboratories with superior specimens, potentially reducing false-negative results without requiring changes to testing methodology. We observed that flocked nasal swabs performed nearly as well as rayon nasopharyngeal swabs in volunteers, suggesting that the flocked design might enable less invasive sampling without compromising specimen quality. This could be particularly valuable in settings where nasopharyngeal sampling is challenging, such as in young children, uncooperative patients, or mass screening scenarios. An ideal sampling device not only collects cellular material efficiently but also releases it effectively into transport medium. The perpendicular arrangement of nylon fibers in the flocked design appears to facilitate both processes. The hydrophilic nature of the nylon pile creates capillary action that improves sample collection, while the perpendicular orientation allows more complete elution of collected material compared to the absorption and entrapment that may occur with traditional fiber swabs. Our study extends previous findings by demonstrating that the advantage of flocked swabs is maintained across different viral etiologies and patient age groups. This consistency reinforces the robustness of the flocked swab advantage and suggests broad applicability across respiratory diagnostics. The slightly higher discomfort reported with flocked nasopharyngeal swabs, though not statistically significant, may reflect the more efficient cell collection mechanism. However, this minor increase in discomfort seems a reasonable trade-off for the substantially improved specimen quality. Furthermore, the possibility of using less invasive flocked nasal swabs may offer an alternative when patient comfort is a priority. Conclusion Our study provides independent validation of previous research demonstrating that flocked swab design significantly improves the collection of respiratory epithelial cells compared to traditional rayon swabs. Mantacc flocked swabs showed a 2-3 fold increase in cell yield across both volunteer and patient populations, with consistent performance across different sampling sites, age groups, and viral etiologies. The improved specimen quality offered by flocked swabs represents a simple yet effective approach to enhancing respiratory infection diagnosis without modifying laboratory testing procedures. The potential impact on diagnostic sensitivity, particularly for detection methods that rely on infected cells, warrants consideration of flocked swabs as the preferred sampling device for respiratory specimens. Future research should quantify the impact of improved sampling on diagnostic sensitivity for specific respiratory pathogens and evaluate the cost-effectiveness of transitioning to flocked swab technology in various clinical settings. Acknowledgments We thank the laboratory staff and volunteers who participated in this study, and the nursing staff who assisted with patient specimen collection. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. Miraclean Technology Co., Ltd provided swabs for evaluation but had no role in study design, data collection, analysis, interpretation, or manuscript preparation. References 1. Daley P, Castriciano S, Chernesky M, Smieja M. Comparison of flocked and rayon swabs for collection of respiratory epithelial cells from uninfected volunteers and symptomatic patients. J Clin Microbiol. 2006;44:2265-2267. 2. Heikkinen T, Marttila J, Salmi AA, Ruuskanen O. Nasal swab versus nasopharyngeal aspirate for isolation of respiratory viruses. J Clin Microbiol. 2002;40:4337-4339. 3. Landry ML, Cohen S, Ferguson D. Impact of sample type on detection of influenza A virus by cytospin-enhanced immunofluorescence and membrane enzyme-linked immunosorbent assay. J Clin Microbiol. 2000;38:429-430. 4. Macfarlane P, Denham J, Assous J, Hughes C. RSV testing in bronchiolitis: which nasal sampling method is best? Arch Dis Child. 2005;90:634-635. 5. Chernesky M, Castriciano S, Jang D, Smieja M. Use of flocked swabs and a universal transport medium to enhance molecular detection of Chlamydia trachomatis and Neisseria gonorrhoeae. J Clin Microbiol. 2006;44:1084-1086. Related posts Everything You Need To Know About Flocked Swabs
READ MORE
02-19-2025
Influenza Diagnosis and Treatment Guidelines (2025 Edition) Part 3
Influenza Diagnosis and Treatment Guidelines (2025 Edition) Part 3 IX. Differential Diagnosis (A) Common Cold The common cold primarily presents with upper respiratory catarrhal symptoms (e.g., rhinorrhea, nasal congestion), with mild systemic symptoms such as fever and myalgia. (B) SARS-CoV-2 Infection SARS-CoV-2 infection shares similar clinical manifestations with influenza and requires differentiation via etiological testing. (C) Other Lower Respiratory Tract Infections When pneumonia is present, differentiation from pneumonia caused by other pathogens (e.g., other respiratory viruses, Mycoplasma pneumoniae) is necessary through etiological testing. X. Treatment (A) General Principles 1. Isolation: Manage patients under respiratory isolation protocols. 2. Hospitalization Criteria (meet any of the following): - Worsening underlying conditions (e.g., COPD, diabetes, chronic heart/kidney failure, cirrhosis). - Meeting criteria for severe or critical influenza. 3. Home Care for Non-Hospitalized Patients: - Isolate at home with room ventilation and mask use. - Ensure adequate rest, hydration, and nutrient-rich diets. - Monitor disease progression closely, especially in children and the elderly. 4. Early Antiviral Therapy for High-Risk Groups: Initiate antivirals promptly in patients at high risk for severe/critical illness to reduce symptoms, complications, duration, and mortality. 5. Antibiotic Stewardship: Avoid inappropriate antibiotic use. Monitor disease progression, collect specimens for etiological testing, and use antibiotics judiciously. 6. Antipyretic Use: Select antipyretics appropriately. Aspirin or aspirin-containing salicylate products are contraindicated in children. (B) Symptomatic Management - Fever: Physical cooling and antipyretics. - Cough/Sputum: Administer antitussives and expectorants. - Oxygen Therapy: Provide oxygen based on hypoxia severity. (C) Antiviral Therapy Antiviral treatment should prioritize initiating therapy within 48 hours of symptom onset for high-risk patients during influenza season, preceded by pathogen testing to confirm infection. For individuals presenting beyond 48 hours, antiviral therapy remains critical for high-risk groups or severe/critical cases with confirmed influenza, as well as for those at risk of transmitting the virus to vulnerable populations. Treatment duration may be extended for severe or critical cases based on pathogen analysis, while combination therapy using agents with identical mechanisms or dose escalation must be avoided. Approved antiviral agents in China include neuraminidase inhibitors (NAIs), RNA polymerase inhibitors, and hemagglutinin inhibitors. Oseltamivir, available in capsule or granule form, is administered at 75 mg twice daily for adults, while pediatric dosing is adjusted by age and weight: children under 1 year receive 3.0–3.5 mg/kg twice daily (stratified by months of age), and those ≥1 year receive 30–75 mg twice daily based on weight categories (≤15 kg to >40 kg), with a standard 5-day course and renal dose adjustments. Intravenous peramivir is dosed at 300 mg (600 mg for severe cases) infused over ≥30 minutes, repeatable daily for up to 5 days in adults, while pediatric dosing follows 10 mg/kg daily (maximum 600 mg) with renal function considerations. Inhaled zanamivir, contraindicated in asthma or chronic respiratory diseases, is prescribed as 10 mg every 12 hours for 5 days in patients ≥7 years. RNA polymerase inhibitors include baloxavir marboxil, given as a single weight-based oral dose (80 mg for ≥80 kg, 40 mg for 20–80 kg, and 2 mg/kg for <20 kg) for individuals ≥5 years, and favipiravir, which is restricted to adults with refractory influenza at 1600 mg twice daily on Day 1 followed by 600 mg twice daily on Days 2–5, strictly contraindicated in pregnancy. The hemagglutinin inhibitor arbidol is prescribed at 200 mg three times daily for 5 days. These regimens emphasize precision in dosing, contraindication adherence, and patient-specific adjustments to optimize outcomes. (D) Supportive Care for Severe/Critical Cases Management focuses on addressing complications, treating underlying conditions, preventing or treating secondary infections, and providing organ-specific supportive care. Conventional oxygen therapy is indicated for patients with a PaO2/FiO2 ratio ≤300. For those with a PaO2/FiO2 ratio ≤200, high-flow nasal cannula (HFNC) or non-invasive ventilation (NIV) should be initiated, accompanied by prone positioning when feasible. Mechanical ventilation is required for patients with a PaO2/FiO2 ratio ≤150 or significant inspiratory effort, particularly in children, and must adhere to lung-protective strategies. Refractory respiratory failure may necessitate extracorporeal membrane oxygenation (ECMO). Airway clearance techniques, such as vibration, chest oscillation, postural drainage, or bronchoscopy, are recommended to maintain pulmonary hygiene. Continuous monitoring of oxygenation and ventilation parameters is critical. Patients with sepsis or shock require hemodynamic stabilization through fluid resuscitation and vasopressor therapy. Close monitoring of blood pressure, heart rate, urine output, and arterial lactate levels is essential. Cardiac biomarkers and electrocardiograms (ECG) should be assessed regularly to detect myocardial injury, which may arise from direct viral effects or exacerbation of pre-existing cardiovascular disease. AKI management involves correcting hypoperfusion and discontinuing nephrotoxic agents. Continuous renal replacement therapy (CRRT) is indicated for hyperkalemia, severe metabolic acidosis, or fluid overload unresponsive to diuretics. For encephalitis or encephalopathy, interventions target cerebral edema reduction and seizure control. Acute necrotizing encephalopathy (ANE) should be managed according to the 2023 pediatric ANE guidelines. Acute disseminated encephalomyelitis (ADEM) and transverse myelitis warrant corticosteroids and/or intravenous immunoglobulin (IVIG), while Guillain-Barré syndrome is treated with IVIG or plasma exchange. Systemic corticosteroids are not routinely recommended but may be considered for refractory septic shock after risk-benefit analysis. Nutritional support and early rehabilitation are integral to recovery, tailored to the patient’s metabolic needs and functional status. XI. Hospital Infection Control 1. Triage System: Implement pre-examination triage, enforce mask use for symptomatic patients/visitors, and promote hand/respiratory hygiene. 2. Isolation: Separate suspected/confirmed cases. Restrict visits and ensure mask use during transfers. 3. Ventilation and Disinfection: Maintain airflow and clean high-touch surfaces (wards, clinics, offices). 4. Waste Management: Dispose of medical waste properly; perform terminal disinfection post-discharge. 5. Staff Protection: Follow standard precautions (surgical masks, hand hygiene). Screen symptomatic staff and exclude infected personnel from work. XII. Prevention (A) Vaccination ✅Most effective measure to reduce infection and complications. Recommended for all ≥6 months without contraindications. ✅Priority Groups: Healthcare workers, elderly ≥60 years, chronic disease patients, pregnant women, children 6–59 months, caregivers of infants <6 months, and congregate settings (schools, prisons). (B) Chemoprophylaxis ✅Post-Exposure Prophylaxis: For high-risk close contacts (unvaccinated or unimmunized) within 48 hours of exposure. (C) General Measures ✅Hygiene Practices: Frequent handwashing, ventilation, avoiding crowded areas, and masking in public if symptomatic. ✅Respiratory Etiquette: Cover coughs/sneezes with elbow/tissue; avoid touching face. ✅Self-Isolation: Rest and isolate if symptomatic; wear masks during medical visits. About Us: For efficient virus isolation, high-quality oropharyngeal swabs are essential. Mantacc produces medical-grade, sterile, and DNase/RNase-free flocked swabs compatible with viral transport media and molecular diagnostics, certified to ISO, CE, and FDA standards. Learn more at Mantacc 93050L Oral Sampling Swabs. Related Post Influenza Diagnosis and Treatment Guidelines (2025 Edition) Part 1 Influenza Diagnosis and Treatment Guidelines (2025 Edition) Part 2 Oral Sampling Swabs: A Promising Alternative for Infectious Disease Diagnosis
READ MORE
02-19-2025
Influenza Diagnosis and Treatment Guidelines (2025 Edition) Part 2
Influenza Diagnosis and Treatment Guidelines (2025 Edition) Part 2 V. Laboratory and Imaging Findings (A) General Laboratory Tests 1. Complete Blood Count (CBC): White blood cell (WBC) count is typically normal or decreased. Severe cases may show a significant reduction in lymphocyte count. 2. Blood Biochemistry: Elevated levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), lactate dehydrogenase (LDH), and creatinine may occur. A minority of cases show increased creatine kinase (CK). Electrolyte imbalances, such as hypokalemia, may be present. Shock cases may exhibit elevated blood lactate. 3. Arterial Blood Gas Analysis: Severe cases may demonstrate decreased partial pressure of oxygen (PaO2), oxygen saturation (SaO2), and oxygenation index (PaO2/FiO2), along with acid-base imbalance. 4. Cerebrospinal Fluid (CSF): In central nervous system (CNS) involvement, cell counts and protein levels may be normal or elevated. Acute necrotizing encephalopathy (ANE) typically shows normal cell counts with elevated protein. (B) Etiological Testing 1. Antigen Detection: Nasopharyngeal or pharyngeal swab antigen testing is rapid and simple but less sensitive than nucleic acid testing. A positive result supports diagnosis, but a negative result does not exclude influenza. 2. Nucleic Acid Testing: Nasopharyngeal swabs, pharyngeal swabs, tracheal aspirates, sputum, or bronchoalveolar lavage fluid can be tested via nucleic acid amplification (e.g., RT-PCR), which has high sensitivity and specificity and can distinguish viral types/subtypes. 3. Virus Culture: Influenza virus can be isolated from respiratory specimens. (C) Serological Testing A retrospective diagnosis can be made if convalescent-phase IgG antibodies seroconvert or show a fourfold or greater increase compared to the acute phase. (D) Imaging Findings Primary viral pneumonia: Imaging reveals lung patchy shadows, ground-glass opacities (GGO). Rapid progression may lead to bilateral diffuse infiltrates or consolidations. Rare cases show pleural effusion. Acute necrotizing encephalopathy (ANE): CT or MRI may show multifocal brain lesions, including thalamus, periventricular white matter, internal capsule, putamen, dorsal upper brainstem (around the fourth ventricle and ventral midbrain aqueduct), and cerebellar medulla. Bilateral symmetrical thalamic lesions are characteristic. VI. Diagnosis Diagnosis is based on epidemiological history, clinical manifestations, and etiological testing. During influenza season, even with atypical symptoms, influenza should be considered for high-risk or hospitalized patients, and etiological testing is required. During non-epidemic periods, influenza testing should be performed for hospitalized patients with suspected viral pneumonia, in addition to testing for common respiratory pathogens. (A) Clinically Diagnosed Cases Patients with epidemiological history (close contact with suspected/confirmed influenza cases within 7 days before onset without effective protection, belonging to a cluster of influenza-like cases, or having clear evidence of transmission) and typical influenza symptoms, after excluding other causes of influenza-like illness. (B) Confirmed Cases Patients with influenza symptoms and at least one positive etiological test: 1. Positive influenza antigen test. 2. Positive influenza nucleic acid test. 3. Positive influenza virus culture. 4. IgG antibody seroconversion or a fourfold or greater increase in convalescent-phase titers. VII. Clinical Classification (A) Mild Type Manifested as upper respiratory tract infection. (B) Moderate Type Fever >3 days and/or cough, shortness of breath, but respiratory rate (RR) <30 breaths/min and oxygen saturation (SpO2) >93% at rest on room air. Imaging shows pneumonia. (C) Severe Type 1. Adults meeting any of the following: ✅Tachypnea (RR ≥30 breaths/min). ✅SpO2 ≤93% at rest on room air. ✅PaO2/FiO2 ≤300 mmHg (corrected for altitude >1000m using: PaO2/FiO2 × [760/atmospheric pressure (mmHg)]; 1 mmHg = 0.133 kPa). ✅Rapid clinical worsening with >50% progression of lung lesions on imaging within 24–48 hours. 2. Children meeting any of the following: ✅Extremely high fever or persistent fever >3 days. ✅Tachypnea (≥60 breaths/min for <2 months; ≥50 breaths/min for 2–12 months; ≥40 breaths/min for 1–5 years; ≥30 breaths/min for >5 years), excluding fever/crying effects. ✅SpO2 ≤93% at rest on room air. ✅Nasal flaring, chest retractions, wheezing, or dyspnea. ✅Altered consciousness or seizures. ✅Refusal to eat, feeding difficulties, or signs of dehydration. (D) Critical Type Patients meeting any of the following: 1. Respiratory failure requiring mechanical ventilation. 2. Shock. 3. Acute necrotizing encephalopathy (ANE). 4. Multi-organ failure requiring ICU care. VIII. High-Risk Populations for Severe/Critical Illness The following groups are at higher risk for severe/critical influenza and require early antiviral treatment and monitoring: 1. Children <5 years (highest risk <2 years). 2. Adults ≥65 years. 3. Individuals with chronic conditions: respiratory, cardiovascular (excluding hypertension), renal, hepatic, hematologic, neurological/neuromuscular, metabolic/endocrine disorders, malignancy, or immunosuppression. 4. Obese individuals. 5. Pregnant and postpartum women. To be continued... About Us: For efficient virus isolation, high-quality oropharyngeal swabs are essential. Mantacc produces medical-grade, sterile, and DNase/RNase-free flocked swabs compatible with viral transport media and molecular diagnostics, certified to ISO, CE, and FDA standards. Learn more at Mantacc 93050L Oral Sampling Swabs. Related Post Influenza Diagnosis and Treatment Guidelines (2025 Edition) Part 1 Influenza Diagnosis and Treatment Guidelines (2025 Edition) Part 3 Oral Sampling Swabs: A Promising Alternative for Infectious Disease Diagnosis
READ MORE


.webp?x-oss-process=image/resize)