HPV Test Sampling Swabs: Pros, Cons and Guidance
What are the benefits and challenges of using swabs for HPV self-sampling?
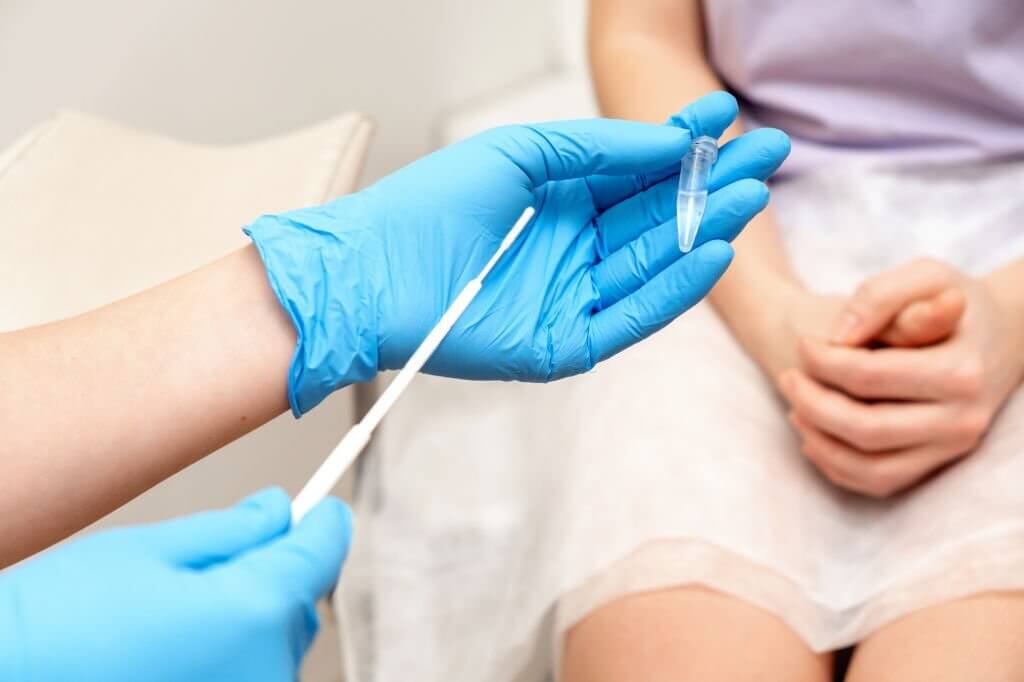
Self-sampling for HPV testing has emerged as an important strategy to improve participation and access to cervical cancer screening. Allowing women to collect their own vaginal sample rather than needing a pelvic exam by a clinician can help overcome barriers like discomfort, embarrassment, scheduling difficulties and transportation issues. But what are the best devices and methods for self-sampling? Swabs present some advantages but also raise questions around accuracy and usability.
What types of swabs can be used for HPV self-sampling?
A variety of swabs have been evaluated for self-collection of vaginal samples for HPV testing, including cotton, flocked nylon, polyester and dacron. Cotton and flocked swabs are among the most common. Flocked swabs use smallfiber tufts that are designed to improve sample collection and release. Some key factors in swab selection include cost, comfort, efficiency in sampling cells from the vaginal walls and cervix, and release of material into the sample medium. The specific swab used may depend on the available HPV test and what it has been validated for.
How accurate are HPV results from self-collected swab samples?
Multiple studies have found good agreement between HPV test results from clinician-collected cervical samples and self-collected vaginal samples using various swabs. A systematic review and meta-analysis published in 2014 concluded that vaginal self-sampling showed similar sensitivity and specificity to detect underlying high-grade cervical lesions compared to clinician sampling, for PCR-based HPV assays.
Some studies have suggested that flocked swabs may collect more cellular material and yield somewhat higher analytical sensitivity for HPV compared to regular cotton swabs. However, other analyses found little difference. Overall, both cotton and flocked nylon swabs appear capable of collecting adequate samples for accurate HPV detection if used properly.
What are the benefits of using swabs for self-sampling?
-
- Simple and affordable collection method. Swabs are inexpensive, easily obtained and familiar to use.
-
- Comfortable for self-collection. Swabs are slender and smooth, minimizing discomfort during insertion and collection.
-
- Dry storage possible. Swab samples can be stored dry and transported at ambient temperature if needed, avoiding costs and hazards of transport medium.
-
- Flexible usage. Women can collect swab samples themselves at home, a clinic or other location, providing more options to fit personal preferences.
-
- Established processing methods. Many existing HPV tests and labs are already set up to extract and test material from cervical or vaginal swabs.
What are some of the limitations or challenges with using swabs?
-
- Sample volume may be low. Swabs only absorb a limited amount of fluid and cells compared to a lavage or brush. Their capacity and release may affect DNA yield.
-
- Sample quality and stability. Dry swab samples may be more prone to DNA degradation over time versus samples stored in liquid medium. This could lead to lower sensitivity or invalid results.
-
- Proper technique is key. Rotating the swab during insertion and sampling the vaginal walls is important to collect shed cervical cells. Women need clear instructions.
-
- Differences in swab types. Not all swabs have been clinically validated with the particular HPV test used. Confirmation is needed that the specific swab-test combination works.
-
- Breakage and contamination risks. Cotton swabs may bend or break off during sample collection. The sampled tip should not contact any surface before going into the tube.
What guidance exists for using swabs in self-sampling?
The World Health Organization (WHO) has issued recommendations supporting the use of self-sampling as an alternative means to increase participation in cervical cancer screening programs. In WHO guidelines published in 2021 on nucleic acid amplification tests for screening, as well as 2022 guidelines on self-care interventions, the organization recommends offering self-sampling with validated HPV tests as an additional approach along with provider-collected cervical samples. WHO states dry swabs or brushes are among the potential self-collection devices, though they note that self-collected vaginal samples tend to have lower sensitivity compared to clinician-taken cervical samples. Importantly, WHO guidance emphasizes that self-sampling strategies must use PCR-based tests that have been clinically validated specifically for use with self-collected vaginal samples, and that programs need to have systems to ensure follow-up management for women who screen HPV positive. WHO's recommendations lend further global endorsement to exploring implementation of self-sampling to expand cervical screening access, while underscoring the need to adhere to quality assurance and follow-up standards.
Should programs start incorporating self-sampling swabs?
Where resources and infrastructure permit HPV testing, introducing opt-in self-sampling using validated swabs could help expand screening coverage and reduce cervical cancer burden. Ensuring quality specimen collection, affordableprocessed kits and effective follow-up for those with positive results are critical to realizing the benefits. Ongoing monitoring and evaluation of swab self-sampling implementation, costs, feasibility, and users’ experiences will further guide best practices.
Click to View → Mantacc HPV Test Sampling Swabs, Mantacc 95000LV Cervical Swab
References
-
1. Yeh PT, Kennedy CE, de Vuyst H, Narasimhan M. Self-sampling for human papillomavirus (HPV) testing: a systematic review and meta-analysis. BMJ Glob Health. 2019 May 14;4(3):e001351. doi: 10.1136/bmjgh-2018-001351. PMID: 31179035; PMCID: PMC6529022.
-
2. Nishimura H, Yeh PT, Oguntade H, Kennedy CE, Narasimhan M. HPV self-sampling for cervical cancer screening: a systematic review of values and preferences. BMJ Glob Health. 2021 May;6(5):e003743. doi: 10.1136/bmjgh-2020-003743. PMID: 34011537; PMCID: PMC8137189.
-
3. Vassilakos, P., Catarino, R., Bougel, S. et al. Use of swabs for dry collection of self-samples to detect human papillomavirus among Malagasy women. Infect Agents Cancer 11, 13 (2016). https://doi.org/10.1186/s13027-016-0059-8
-
4. WHO recommendations on self-care interventions, Human papillomavirus (HPV) self-sampling as part of cervical cancer screening and treatment, 2022 update