Everything You Need to Know about Colorectal Cancer Screening
Colorectal cancer is one of the most common cancers worldwide. Most colorectal cancers develop from benign polyps (adenomas and serrated polyps) through a series of genetic and epigenetic changes over 10-15 years. Colorectal polyps are extremely common; about half of people over 50 have polyps. Therefore, even though nearly all colorectal cancers arise from polyps, only a small fraction of polyps progress to cancer.
Removing colon polyps when found through colonoscopy can halt the progression to colorectal cancer. Screening to find and remove colorectal adenomas reduces the incidence of colorectal cancer and thus decreases mortality from colorectal cancer. In addition, screening can detect cancer at an early stage, which also lowers mortality.
A recent review published in January 2023 in NEJM Evidence systematically reviewed methods, evidence, and future directions for colorectal cancer screening. Here we summarize the key points.
Screening
Screening is done in healthy people without clinical signs or symptoms, unlike testing in symptomatic patients. Because screening targets healthy individuals, it must provide a net benefit. The benefits, harms, and burdens of any screening test should be considered before recommending it.
Cancer screening typically involves multiple steps (Figure 1). There is an initial test, with positive results leading to further evaluation to make a definitive diagnosis, followed by treatment of diagnosed disease or precancerous lesions. Those with negative screening results usually need repeated screening at intervals, such as annual or biennial mammography for breast cancer screening or periodic stool testing for colorectal cancer screening.
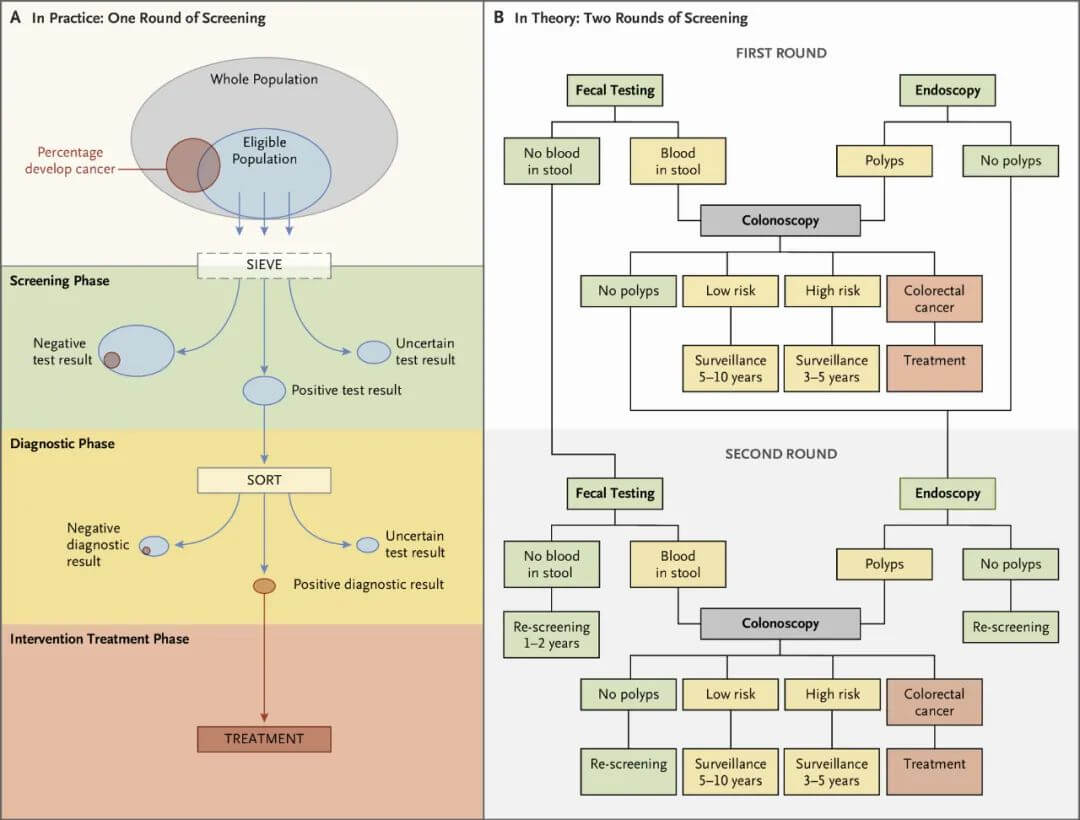
Figure 1. Steps in colorectal cancer screening.
There are two distinct concepts in cancer screening: prevention screening and early cancer detection screening. The screening approaches and performance are markedly different. Prevention screening aims to detect benign precancerous lesions. Early cancer detection screening does a poor job of detecting benign precancers and instead aims to detect invasive cancers at an early stage.
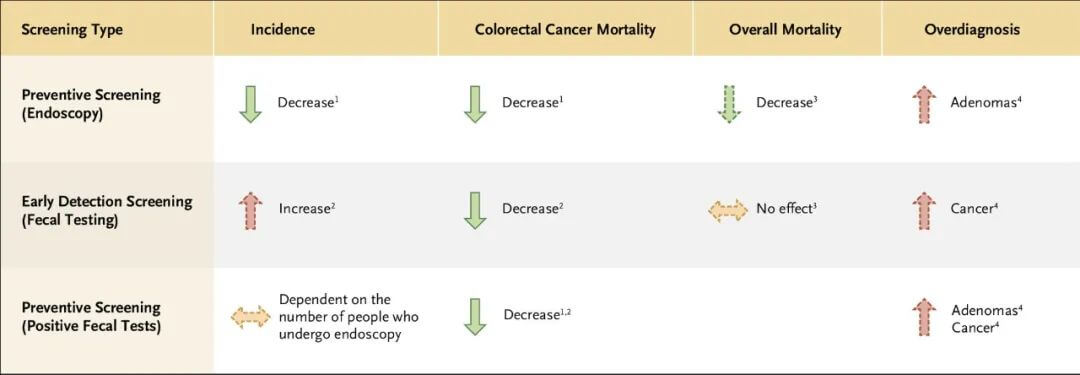
Figure 2. Key features of colorectal cancer screening tools.
Prevention Screening
Identifying and removing benign precancerous lesions prevents invasive cancers. Common prevention screening includes colonoscopy and sigmoidoscopy for colon cancer and Pap smears for cervical cancer. The primary goal of prevention screening is to avert cancers from occurring. Thus, these modalities work by lowering cancer incidence. Although most prevention screening can also detect early cancers, the reduction in cancer mortality is predominantly due to lower cancer incidence. It takes a long time, significantly longer than the time for progression from early invasive to late cancer, for benign precancers to develop into invasive cancers, so the interval between rounds of prevention screening can be quite long.
Early Cancer Detection Screening
Most cancers lack well-defined precancerous lesions or current methods to detect them. Thus, most cancers cannot undergo prevention screening. Mammography for breast cancer and prostate-specific antigen (PSA) screening for prostate cancer are examples of early cancer detection screening.
Detecting cancer early to lower mortality requires screening to find cancers at an earlier stage compared to no screening. Early cancer detection screening does not lower cancer incidence. In fact, mammography and PSA screening detect some small tumors that would never grow, cause symptoms, or result in death, thus increasing cancer incidence – one of the harms of cancer screening termed “overdiagnosis.”
Colorectal Cancer Screening Tests
Several methods are available for colorectal cancer screening. To date, only guaiac-based fecal occult blood testing (gFOBT) and sigmoidoscopy have been shown in randomized trials to reduce colorectal cancer incidence and associated mortality. However, the most commonly used screening modalities are fecal immunochemical testing (FIT) and colonoscopy.
Large randomized trials comparing FIT to colonoscopy or comparing colonoscopy to no screening are underway with results expected in 2022. In the interim, results from observational studies and models suggest FIT and colonoscopy can lower colorectal cancer incidence and associated mortality. The major limitation of observational studies and models is non-random screening assignment – essentially comparing colorectal cancer incidence and associated mortality between people who chose to get screened versus not. It is well known that screeners and non-screeners often differ drastically in cancer risk, associated mortality, comorbidities, and socioeconomic status, leading to self-selection bias in non-randomized screening trials. While observational studies and models are limited in estimating the efficacy of FIT and colonoscopy screening, it is reasonable to infer they should be at least as good as gFOBT and sigmoidoscopy.
Other emerging colorectal cancer screening technologies – CT colonography, capsule endoscopy, or newer stool or blood-based biomarker tests – may play a role in future screening but most are not in widespread use.
Fecal Testing
Fecal testing is a stepped approach requiring repeated testing at intervals. All those testing positive undergo colonoscopy to establish a diagnosis and remove any detected polyps.
Fecal occult blood testing is an early cancer detection approach. It is based on the premise that early colorectal cancers bleed, allowing detection of small amounts of blood in the stool before symptoms develop. Due to the intermittent nature of bleeding from cancers, fecal testing has limited sensitivity, necessitating repeated testing. The optimal interval and number of tests per round, as well as the age to start and stop screening, remain debated. Most guidelines currently recommend annual or biennial fecal testing.
Causes of a positive fecal test include bleeding polyps, inflammation, peptic ulcers, or hemorrhoids. While fecal screening works primarily by detecting early cancer, it also aids cancer prevention since those testing positive undergo colonoscopy, which can detect and remove polyps.
Previous fecal testing relied on guaiac-based tests, but has been replaced by FITs that are more specific for colorectal disease and quantitatively measure fecal hemoglobin. FITs allow adjustable hemoglobin thresholds to tune test sensitivity and specificity for colorectal cancer and adenomas.
Endoscopic Screening
Endoscopic screening can be done by sigmoidoscopy or colonoscopy. Colonoscopy is the predominant endoscopic screening modality. The goal of endoscopic screening is to find and remove adenomas and serrated polyps to prevent colorectal cancer. Early cancer detection is an additional benefit.
Sigmoidoscopy is less invasive than colonoscopy, requires no sedation, and only limited bowel preparation with an enema. Colonoscopy necessitates more extensive bowel cleansing, takes longer, and often requires sedation or general anesthesia. Beyond its use for primary screening, colonoscopy also serves as follow-up for those with a positive result from another colorectal cancer screening test.
Emerging Screening Tests
Some U.S. guidelines currently recommend fecal biomarker screening, but uptake is limited elsewhere given high costs and limited evidence for reducing colorectal cancer incidence and associated mortality.
Numerous companies and academic centers are developing and evaluating new blood-based screening tests utilizing genetic, epigenetic, or proteomic markers, but none are approved for colorectal cancer or polyp screening yet.
CT colonography is recommended in some U.S. guidelines as a second-line screening option. However, due to high costs, radiation exposure, and the need to follow up all positives with colonoscopy, it is not used for population-based screening.
Post-Polypectomy Surveillance
Patients who have had polyps removed during screening are stratified as high or low risk for future colorectal cancer based on polyp size, number, and histology. Most undergo follow-up colonoscopy, for example every 3, 5 or 10 years depending on polyp characteristics. Surveillance recommendations after polypectomy differ somewhat between regions with more intensive follow-up in the U.S. compared to Europe.
There is a lack of high-quality studies guiding surveillance recommendations after colorectal cancer screening. All current recommendations are based on low-quality evidence. A large European randomized trial of 20,000 patients is underway comparing different surveillance strategies. A similar study sponsored by the U.S. National Institutes of Health will initiate soon.
Benefits and Harms
The balance of benefits and harms of colorectal cancer screening depends on the screening method, with colonoscopy conferring greater absolute benefit than sigmoidoscopy or FIT/gFOBT. However, the degree of benefit depends more on an individual’s cancer risk rather than the screening method. For those with a 2% risk of colorectal cancer, screening 1,000 people prevents 0-7 cancers and averts 1-3 colorectal cancer deaths.
For those with a 4% risk, screening 1,000 people prevents 1-13 cancers and averts 2-7 colorectal cancer deaths.
All tests have adverse effects; bleeding and perforation are the most common serious events with endoscopic exams. The number of adverse events depends on the number of endoscopic exams, which can be for primary screening, follow-up of a positive FIT or sigmoidoscopy, or surveillance. Adverse events increase with age, number of comorbidities, and number of polypectomies.
Overdiagnosis
Overdiagnosis in cancer screening refers to the detection of lesions (like polyps or cancers) that would never cause symptoms or death in the person's lifetime (or polyps that would not progress to cancer). Importantly, overdiagnosis is not the same as a false positive.
It is currently difficult to determine which individual cases represent overdiagnosis since available screening tests cannot distinguish overdiagnosed disease from non-overdiagnosed disease. The exact risk of overdiagnosis from colorectal cancer screening is unknown but is believed to be smaller than that for prostate cancer screening.
Overdiagnosis leads to unnecessary treatment, psychological harm (e.g. anxiety), and economic costs. Additionally, screening can lead to unnecessary monitoring and follow-up, potentially causing further overdiagnosis and harm.
Both prevention and early detection screening confer some degree of overdiagnosis risk. The consequences of overdiagnosed cancer are more severe than overdiagnosed polyps since cancer treatment is more invasive than polyp removal. However, given the far larger number of polyp than cancer patients, even overdiagnosed polyps in prevention screening can cause considerable population harm that policymakers and screening providers should consider. In the general screening population at age 60, adenoma prevalence is 32% whereas lifetime colorectal cancer risk for Americans is about 4.2%. Thus, most adenomas will not progress to cancer.
Quality
Success of colorectal cancer prevention screening relies on reliable polyp detection. In the last decade, there is increasing recognition of wide variation in endoscopists’ abilities to comprehensively detect and remove polyps. An individual whose colonoscopy is performed by a high-adenoma detecting endoscopist will have a much lower subsequent colorectal cancer risk compared to someone scoped by a low detector. Consequently, many colorectal cancer screening programs instituted rigorous quality assurance initiatives including training, monitoring, and auditing.
Recently, artificial intelligence (AI) assisted polyp detection during colonoscopy has been introduced to improve adenoma detection rates. Early studies show AI-assisted polyp detection increases average adenoma detection rates from 25% to 37%. AI devices have not improved detection of larger and advanced polyps, those at highest risk of developing cancer. Whether AI-assisted detection of small polyps better prevents colorectal cancer remains unknown.
Guidelines
Clinical practice guidelines have become important tools to guide clinical practice but can also have negative impacts and introduce ethical challenges. Many countries have developed guidelines regarding colorectal cancer screening, most recommending screening average-risk individuals ages 50-79 years. Recently, the U.S Preventive Services Task Force and American Cancer Society lowered the recommended screening start age to 45 years.
Screening provides tremendous clinical benefit for some but also exposes many to burdens and potential harms. Colorectal cancer screening is not a one-time decision but involves colonoscopy and surveillance for many people over time. Individuals may reasonably value the potential benefits and harms differently, leading some to rationally decline screening. Most prior colorectal cancer screening initiatives recommended screening based on age cut-offs alone, without considering an individual's cancer risk. A recent colorectal cancer screening guideline introduced using risk and benefit thresholds to guide screening recommendations. Based on a 3% 15-year colorectal cancer risk threshold, the expert panel weakly recommended screening for those above 3% risk and no screening below 3% risk. Risk calculators have been developed.
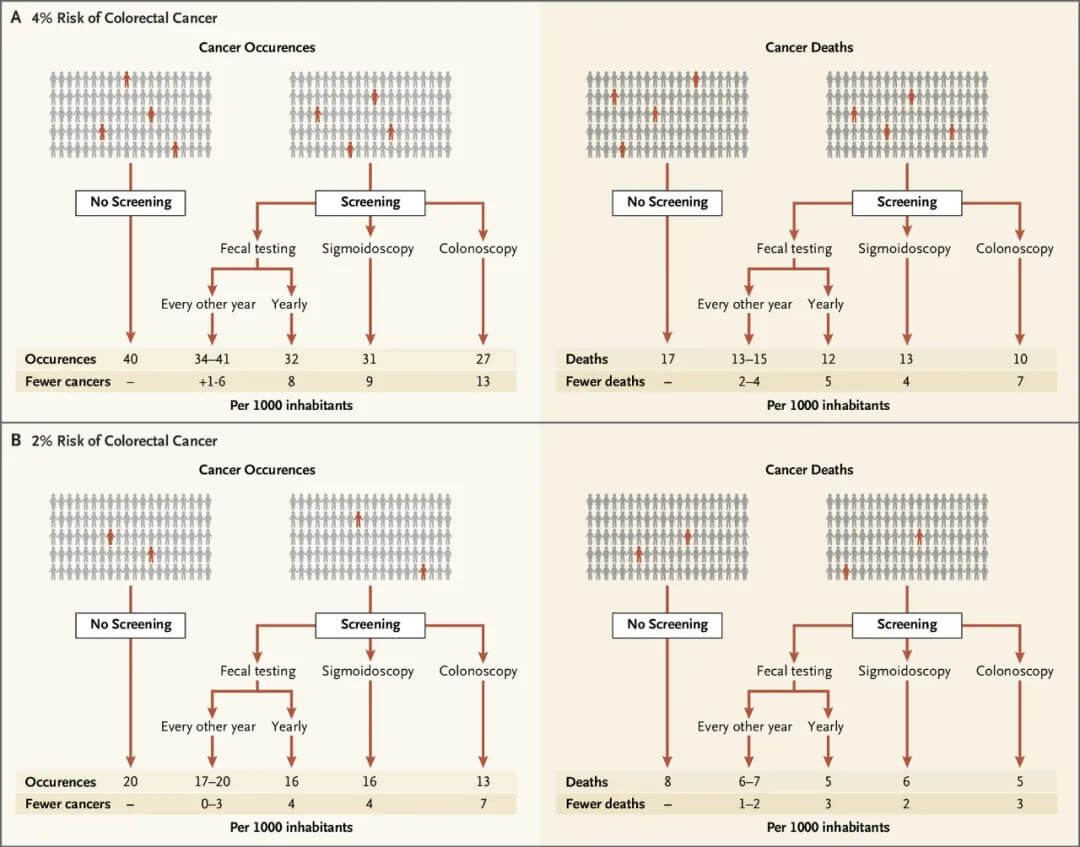
Figure 3. Benefits of different colorectal cancer screening methods.
The Future: Learning Screening Programs
New screening interventions, whether new detection methods or changes in testing frequency, age ranges, or positive thresholds, are best evaluated through clinical trials initially. However, once screening is widespread and most participate, traditional randomized trials become difficult with ineffective control groups to compare benefits and harms of screening.
In the absence of randomized controlled trials, quantifying benefits and harms of prevention services requires new methods like learning healthcare systems to continually and systematically generate knowledge on which detection works best to lower mortality and provides the optimal balance of benefits and harms most likely to be accepted and rapidly implemented.
Conclusion
Colorectal cancer screening allows early detection and/or prevention of cancers with several possible screening methods. FIT and colonoscopy screening have not been tested in randomized trials but are likely at least as good as gFOBT and sigmoidoscopy, which randomized trials have shown reduce colorectal cancer incidence and associated mortality.
The potential absolute benefits of screening vary with an individual’s cancer risk, while harms depend primarily on the number of colonoscopies. The overall balance of benefits and harms is difficult to quantify and guidelines recognize individuals may reasonably value the potential benefits and harms differently. As screening becomes widespread, new and more effective screening strategies, including those involving screening frequency and age ranges, should be systematically and continually tested in learning screening programs.
To increase colorectal cancer screening rates, Mantacc has developed an innovative Stool Sample Collection Kit. This at-home kit enables patients to easily collect and mail stool samples to the lab for analysis using FIT or stool DNA tests. The collection device and preservative solution allow reliable at-home sampling and stabilization of biomarkers. By simplifying sample collection, Mantacc’s user-friendly kit removes barriers to recommended screening. The company aims to increase accessibility and adherence to colorectal cancer screening through this novel approach.
Click to View → Mantacc Stool Sample Collection Kit
References
Helsingen LM, Kalager M. Colorectal cancer screening—Approach, evidence, and future directions. NEJM Evidence 2022;1: EVIDra2100035.