New Guidelines of HPV Testing for Cervical Cancer Screening
The Cancer Prevention and Control Professional Committee of the Chinese Preventive Medicine Association, the Colposcopy and Cervical Lesions Professional Committee of the Obstetrics and Gynecology Branch of the Chinese Medical Doctor Association, the Colposcopy and Cervical Pathology Branch of the China Association of Eugenics, and the Medical Laboratory Physician (Technician) Branch of the Beijing Medical Doctor Association, together with the Editorial Board of the Chinese Medical Journal, invited over 50 experts across public health, obstetrics and gynecology, laboratory medicine, pathology and other disciplines to form an expert consensus based on domestic and foreign guidelines, medical evidence, and China’s actual conditions.
The consensus recommends HPV DNA testing as the primary method for cervical cancer screening in China, and provides scientific suggestions on pre-implementation evaluation and preparation, specific applications and target populations, laboratory procedures, and post-implementation monitoring and evaluation when applied to cervical cancer screening practices.
The main contents of the consensus are in two sections. The first introduces HPV genotypes and nucleic acid testing, with the following recommendations:
Recommendation 1:
(1) Adopt the principle of "equal risk, equal management" for the tiered management of HR-HPV positive cases. HPV16/18 have the highest carcinogenic risk and require timely referral for colposcopy to further assess. More evidence on pathogenic risks needs to be accumulated for other HR-HPV infections, and appropriate triage strategies should be proposed after evaluating triage effectiveness.
(2) The risk of cervical precancerous lesions and cervical cancer increases for those with persistent HR-HPV infection. More active management strategies should be adopted.
Recommendation 2:
HPV nucleic acid detection methods can be divided into non-amplification and amplification methods, which can effectively detect the entire HPV genome or certain fragments (e.g. L1 DNA, E6/E7 DNA or mRNA as detection targets).
The second section covers the clinical significance of HPV genotyping detection for cervical cancer screening, with the following recommendations:
Recommendation 3:
(1) HPV nucleic acid testing laboratories should formulate relevant documentation and management systems for the environment, safety, personnel, equipment, reagents and other aspects, establish standard operating procedures covering the entire detection process, and implement a sound quality control program.
(2) It is recommended to select HPV nucleic acid test reagents or detection systems approved by the NMPA that have passed laboratory analytical performance verification.
(3) When applying HPV nucleic acid test reagents or detection systems for clinical cervical cancer screening, their clinical performance should be validated to evaluate if they meet the expected clinical intended use.
Recommendation 4:
(1) Screening population:
-
1. Prioritize screening for women aged 35-64;
-
2. Incorporate into routine health checks where conditions permit; corporate health checks should also follow this recommendation.
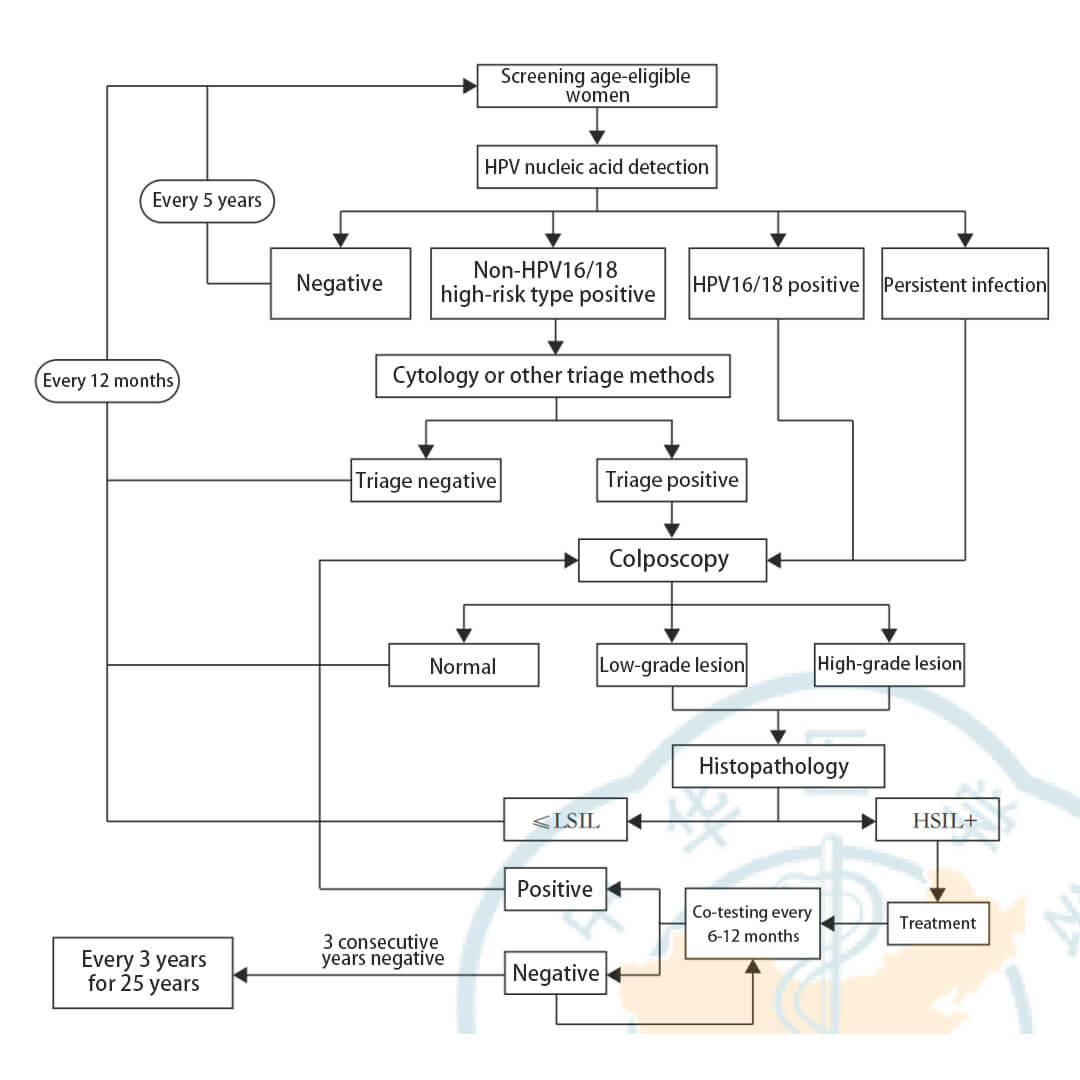
(2) Screening method: Recommend HR-HPV testing as the preferred method for population-based cervical cancer screening, with a screening interval of 5 years.
(3) HPV test selection:
-
1. Detect at least 13 HR-HPV types (HPV16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68);
-
2. Recommend HPV16/18 genotyping where conditions permit;
-
3. Adopt HPV testing methods approved by NMPA that have passed laboratory analytical performance verification and clinical performance verification.
(4) For HPV-positive cases identified during screening, further management recommendations should be provided based on their HSIL+ risk:
-
1. Referral for immediate colposcopy for HPV16/18 positives;
-
2. Triage testing (cytology or other methods) for non-16/18 HR-HPV positives, with referral for colposcopy if triage result is positive;
-
3. Referral for immediate colposcopy for persistent HR-HPV infections.
Recommendation 5:
For ASC-US cytology, HPV DNA testing is recommended as the preferred triage method, and whether referral is required depends on triage results:
(1) Repeat screening in 3 years for HPV negatives. For regions with relatively insufficient cytology physician diagnosis and quality control, the repeat interval can be 12 months.
(2) Immediate referral for colposcopy for HPV positives.
Recommendation 6:
(1) At 6 months after HSIL treatment, the first surveillance comprises standalone HPV DNA testing or joint cytology testing. If results are negative, repeat standalone HPV DNA testing should be performed annually in the next 3 years, and the follow-up interval can be extended to every 3 years if consistently negative up to 25 years.
(2) For AIS patients who have undergone excisional cervical treatment with negative margins and wish to preserve fertility, conservative management can be implemented. Joint cytology and HPV testing, along with cervical canal assessment, is recommended every 6 months in the first 3 years post-operation. If results remain negative, joint cytology and HPV testing along with cervical canal assessment can be performed annually in the next 2 years. If consistently negative in 5 years, the follow-up interval can be further extended to every 3 years, with lifetime follow-up.
(3) For total hysterectomy with prior HSIL+, annual joint cytology and HPV DNA testing is recommended in the first 3 years post-operation. If consistently negative, the follow-up interval can be extended to every 3 years, up to 25 years.
Recommendation 7:
(1) HPV vaccinated population: Recommend adopting the same screening strategies as the unvaccinated population.
(2) Immunocompromised population:
-
1. HIV population: Recommend initiating screening for women within 1 year of being 25 years old or sexually active, with regular screening every 3-5 years. After positive screening results or post-treatment for cervical lesions, the screening interval should be shortened. The specific screening frequency should be discussed and determined with specialists, generally twice as frequent as the general population;
-
2. For other immunocompromised populations, the same screening strategies as the HIV population can be followed.
(3) Pregnant and preparing-for-pregnancy women should follow the same principles as non-pregnant women for screening and management:
-
1. Women preparing for pregnancy should be asked during preconception checkups whether they have undergone cervical cancer screening in the past year;
-
2. For women without prior screening or irregular screening, screening should be performed during preconception checkups or first antenatal checkups; 3 For screening positive pregnant women, note that cervical canal examinations or endometrial scratches/biopsies cannot be performed; excisional examinations should only be considered when cervical cancer is suspected. If HSIL+ is ruled out by relevant examinations, postpone re-screening until postpartum; If CIN2 or CIN3 is suspected or diagnosed, continue monitoring and postpone re-evaluation or treatment until postpartum.
(4) Women aged ≥65 years:
-
1. Those without regular screening in the past 10 years should continue screening;
-
2. If the past 10 years show 3 consecutive normal cytology or 2 consecutive normal HPV screenings with no history of CIN, screening can be stopped.
This consensus reflects the unified views of a multidisciplinary panel of experts drawing upon international best practices, cutting-edge research, and the specific realities of cervical cancer screening in China. As technologies and evidence continue advancing, it will be important to regularly revisit and augment these recommendations. Leveraging high-quality HPV DNA assays is poised to expedite China’s shift toward molecular screening modalities. This transition promises to expand screening access, elevate quality standards, and accelerate progress toward the elimination of cervical cancer nationwide. By sharing the rationale and outcomes shaping China’s screening policies, study authors hope these insights may benefit other global elimination efforts.
Click to View → Mantacc Cervical Swab