Product Features
- High efficacy of broad spectrum germicidal.
- Very low risk of allergic reactions.
- Non-corrosive and ultrasterile.
- Comfortable sponge tip and ergonomic handle.
Products
Company Profile
Life Science
Medical
Environmental
Crime Science
Laboratory Consumables
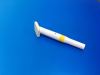
Sponge applicator with 26ml disinfectant containing 2% chlorhexidine gluconate and 70% ethanol for skin preparation. Model: MCA-T260.
Mantacc MCA-T260 26ml CHG Applicator

Surgical Site Infections (SSIs) can be very burdensome to the healthcare system, healthcare workers, and patients:.
1. The average cost per SSI patient is approximately $20,785If the patient has CHG sensitivity, avoid CHG and consider non-alcoholic preparations.
If the preparation area has a high colony count, a separate disinfectant should be used.
Povidone-iodine solutions are recommended for ophthalmic, otologic, mucous membrane and large surface area skin preparations.

Industry: Medical Diagnostics
Total Length: 8.504" (216mm)
Tappet Length: 2.953'' (75mm)
Safe Lock Length: 0.650'' (16.5mm)
Handle Length: 4.902'' (124.5mm)
Tip Length: 2.028'' (51.5mm)
Tip Width: 2.224'' (56.5mm)
Tip Material: PU Sponge
Handle Material: PP
Sterilization: Ethylene Oxide Sterile
Classification: Class Is
Ethylene Oxide Residue: ≤10 µg/g
Storage Conditions: Room temperature
Period of Validity: 36 months

PACKAGING DETAILS: 20/250/1 (250 bags of 20 swabs individually wrapped in paper pouch = 5000 pcs in 1 box)
BOX DIMENSIONS: 18.11 x 12.20 x 10.63 inch (46 x 31 x 27 cm)
PALLET SIZE: 24 or 35 boxes on a pallet
| QUANTITY | DELIVERY TIME |
| ≤10,000 pcs | 3 working days |
| ≤100,000 pcs | 7 working days |
| ≤1,000,000 pcs | 15 working days |
| ≥1,000,000 pcs | please contact us |
Major Organisation Guidelines
1. The CDC guidelines recommend the use of preparations containing 0.5% or more CHG before and after central venous catheter and peripheral arterial catheter insertion.
2. The INS guidelines recommend the use of a solution containing 0.5% or more CHG and alcohol for skin disinfection.
3. Safer Healthcare Now! recommends the use of CHG preparations to significantly reduce infections and should be included in the central venous catheter insertion checklist.
4. The WHO guidelines strongly recommend the use of CHG acetate-based alcohol preparations for surgical site skin preparation.
5. The ORNAC guidelines recommend the use of a solution containing CHG and 70% alcohol for surgical site cleansing.
6. The NICE guideline recommends the use of alcohol-based CHG solutions for skin preparation.
Recommendations for Uses
1. 0.5% CHG/70% IPA: can be used for peripheral venous catheter insertion, central venous catheter maintenance and blood cultures.
2. 2% CHG/70% IPA: may be used for central venous catheter insertion, minor medical procedures, blood cultures, etc.
3. 2% CHG Alcohol-Free Preparation: may be used for peripheral venous catheter insertion, central venous catheter maintenance, blood cultures, etc.
4. Follow manufacturer's instructions for application time and drying time.
5. Select the optimal formulation for each medical procedure.
6. Evaluate the area of skin to be treated; some procedures may require a larger area.